
Therefore, ordering based on the space, the electrons occupy is. The double bonds have 33%, and single bonds have 25% electrons near the nucleus. The triple bonded atoms have 50% electrons present very close to the nuclei than in other regions, increasing the repulsive interactions. The electron density is also highest in triple-bonded atoms, followed by the double and the single bonds. Therefore, lone pairs cause the greatest repulsions. It also considers that such electron-dense regions like lone pair occupy maximum space since it is associated with only one atom’s nuclei, unlike the bond pairs of electrons that share two nuclei. Lone pair-lone pair> lone pair-bond pair> bond pair-bond pair VSEPR takes into account these repulsions and establishes an order as. The lone pair that is not included in the molecular structure influences the shape of NH 3 by bending the bonds from the ideal tetrahedral. However, the molecular structure of NH 3 is not tetrahedral but trigonal pyramidal. NH 3 has 3 bond pairs and one lone pair therefore, electron pair geometry is also four. Its electron pair geometry is 4, and its molecular structure is tetrahedral. The electron-pair geometry is different from the molecular structure since the molecular structure only considers the atom’s location and not the electrons.įor example, comparing CH 4 with NH 3, CH 4 has four electron pairs around the central atom carbon and no lone pairs. VSEPR theory, therefore, calculates electron pair arrangement, also known as electron-pair geometry, to group molecules. When the bonds are fully spread, the repulsion is minimum at 180 o and maximum when the bond angle is around 90 o. The central atom will adopt an arrangement that minimizes the electron pair repulsions by maximizing the distance between them, thereby creating bond angles. The repulsion is maximum if there are lone pair(s) on the central atom. It assumes that once the central atom share electrons with other atoms and forms bonds, the electrons in the bonds start to repel each other.

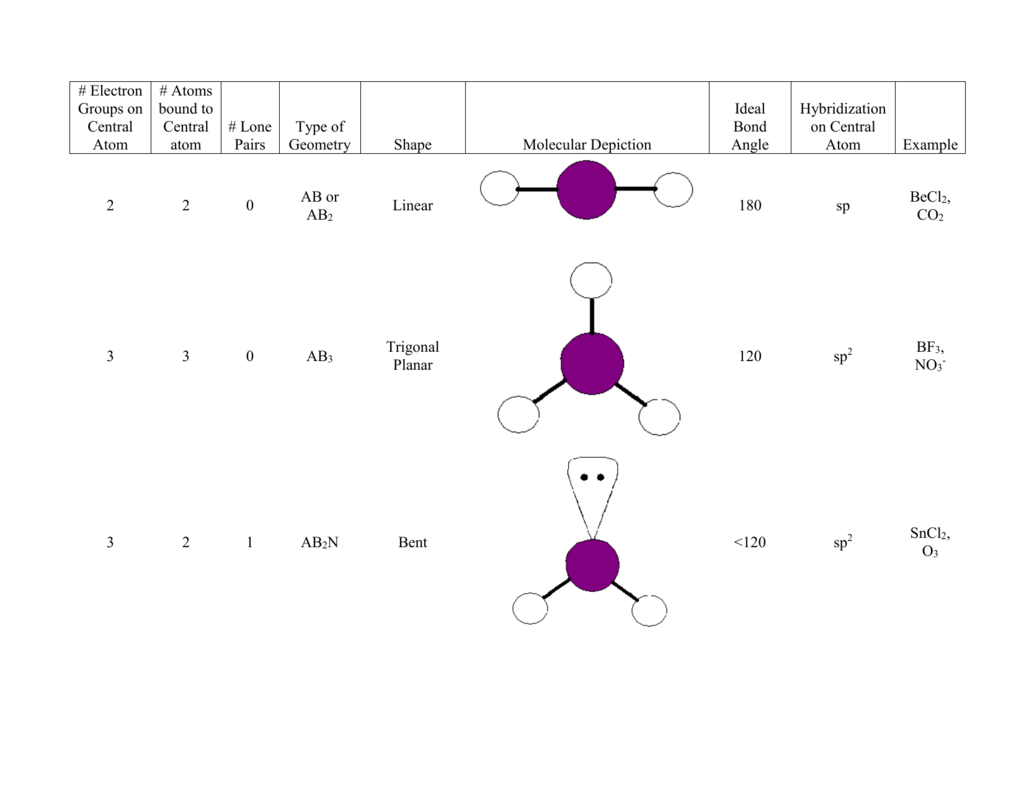
VSEPR theory credits bond angle to a central atom that forms the maximum number of bonds. The VSEPR theory uses electron repulsions occurring between the bonding and the lone pairs post bond formation as a guide to explain the bond angles, molecular geometry, and shape. The premise of VSEPR theory considers shape as an after-effect of bond formation. The first correlation between molecular geometry and valence electrons was presented by Nevil Sidgwick and Herbert Powell in 1940, which was further refined by Ronald Gillespie and Ronald Sydney Nyholm in 1957, known as the Valence shell electron pair repulsion (VSEPR) theory. However, all the structures were drawn planar at right angles without depicting any shape or molecular geometry, which was not true.Ī comparison of the Lewis structure with the actual structure of CH 4 (methane) shows the difference. These bonding electrons are placed in between two atoms, whereas the nonbonding ones are placed above, below, or on the sides. So, two dots were used to represent two-electron covalent bonds or linkages between atoms, the structures popularly called Lewis structures.
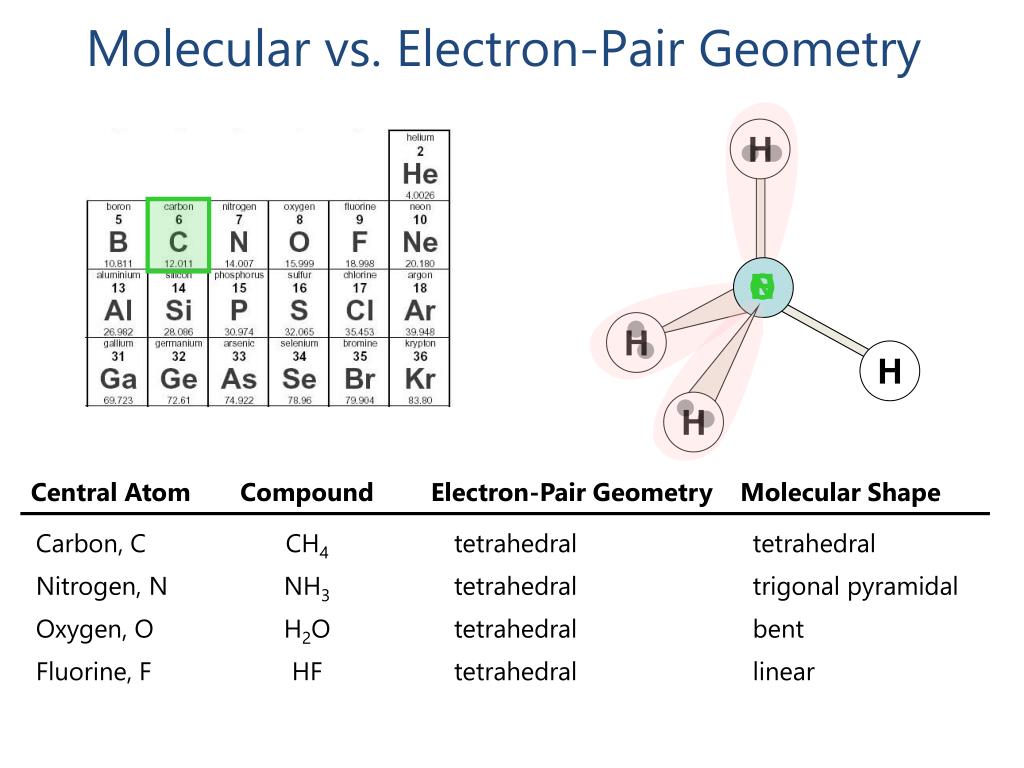
An exception is the Hydrogen atom that attains a duplet configuration.

He used dots to represent an atom’s valence electrons and argued that the atoms share their valence electrons to form one, two, or three bonds until they attain a stable octet electron configuration. Lewis attempted to describe linkages between the atoms to understand the nature of covalent bonds. In his landmark paper, 'The Atom and the Molecule,' G.N.


 0 kommentar(er)
0 kommentar(er)
