

2019 45(1):1-12.Īubron C, Aries P, Le Niger C, Sparrow RL, Ozier Y. The Recurrence Risk of Fetomaternal Hemorrhage. Troìa L, Al-Kouatly HB, McCurdy R, Konchak PS, Weiner S, Berghella V. Impact of initial temporary abdominal closure in damage control surgery: a retrospective analysis. Hu P, Uhlich R, Gleason F, Kerby J, Bosarge P. Retrospective study to investigate fresh frozen plasma and packed cell ratios when administered for women with postpartum hemorrhage, before and after introduction of a massive transfusion protocol. Weiniger CF, Yakirevich-Amir N, Sela HY, Gural A, Ioscovich A, Einav S. The decreased ability to stop bleeding leads to further hypothermia and acidosis, creating a positive feedback loop that results in worsened patient outcomes.Ĭopyright © 2023, StatPearls Publishing LLC. Additionally, dilution of the remaining coagulation components due to volume expanders in addition to hypothermia and acidosis can lead to coagulopathy and altered hemostasis.
At 34 C, effects on coagulation begin, and at 30 C, there is approximately a 50% reduction in platelet activation.Ĭoagulopathy and Dysfunctional Hemostasisĭue to massive bleeding, coagulation factors are often being consumed in patients who may require massive transfusion. Hypothermia reduces the efficacy of both the coagulation cascade (by reducing the enzymatic activity of coagulation proteins) and platelet plug formation. Lower ambient temperatures and decreased blood volume can predispose these patients to hypothermia. Many patients with acute, blood-loss anemia are also susceptible to hypothermia, which again can lead to coagulopathy. This results in delayed and thin fibrin clot formation, which is more quickly destroyed through fibrinolysis.

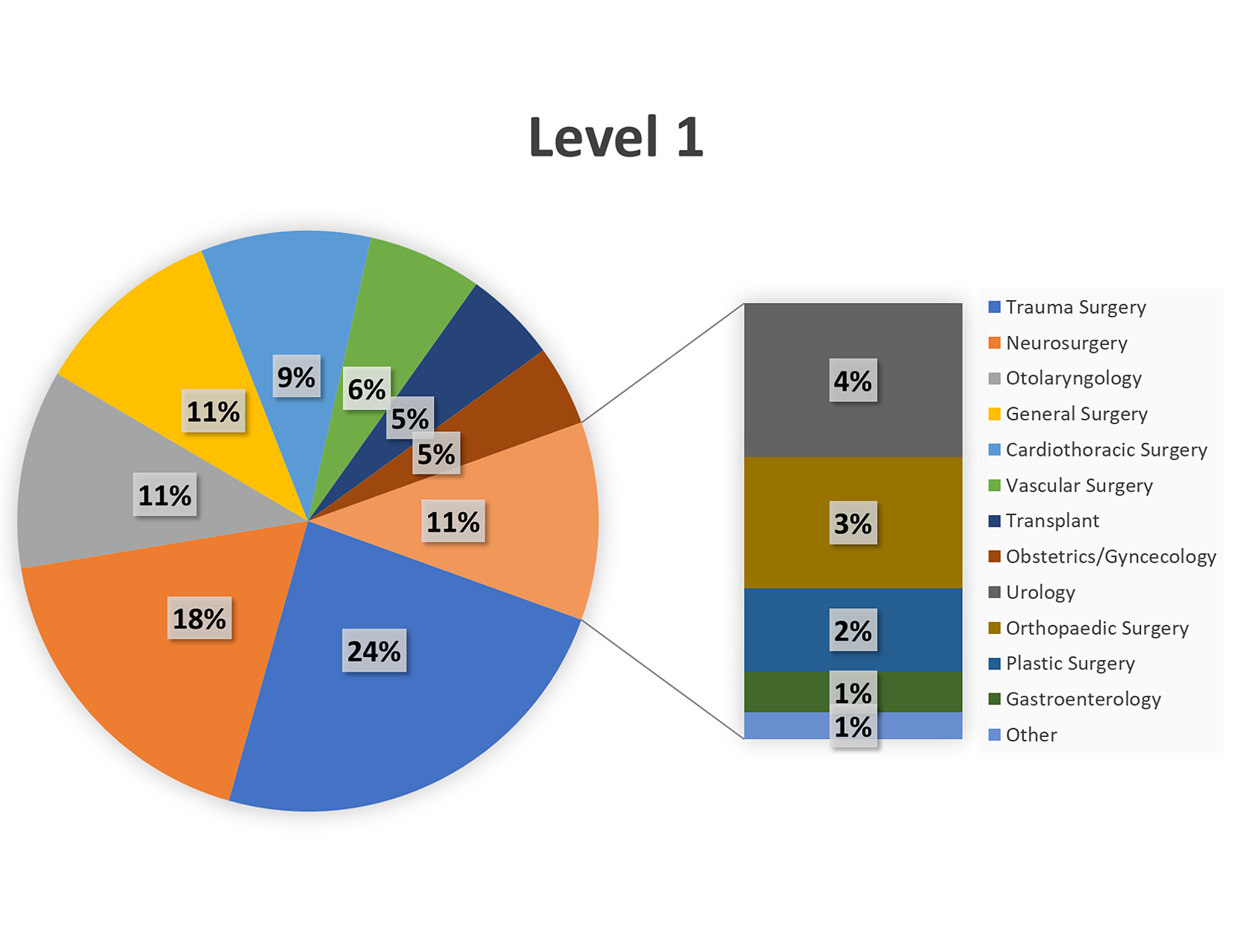
There is a direct relationship between decreasing pH (increasing acidosis) and reduction in the activity of coagulation cascade components. Once acidosis has set in, it further interferes with coagulation by reducing the assembly of coagulation factors. Prolonged states of hypoperfusion lead to acidosis. Patients requiring a massive transfusion are often acidotic even before transfusion begins. Therefore, hemoglobin “thresholds” cannot be used to manage transfusion in the setting of acute blood loss. It is only after time and fluid shifts that the hemoglobin will lower. In acute blood loss, the hemoglobin concentration will remain the same. Hemoglobin is reported as a concentration. However, it is important to remember that these transfusion guidelines are not helpful in the setting of acute blood loss. Ample evidence supports different hemoglobin “thresholds” at which a patient requires transfusion to maintain adequate oxygen delivery. However, if a patient is in severe shock, or if they continue bleeding, blood will eventually be required to maintain oxygen delivery at an appropriate rate.ĭue to the surplus of oxygen delivery in a normal physiologic state, the body is able to maintain tissue oxygenation at below-normal hemoglobin levels. Therefore, volume expanders such as crystalloids may be employed during a massive transfusion to maintain blood pressure and tissue perfusion. At baseline, oxygen is delivered to tissues at approximately four times the rate of oxygen tissue consumption. The focus when a patient is in hypovolemic shock secondary to acute blood loss is to expand intravascular volume and maintain oxygen delivery to tissues. Massive transfusion requires the consideration of multiple physiologic parameters such as volume status, tissue oxygenation, management of bleeding and coagulation abnormalities, and acid-base base balance. While the incidence of massive transfusion is relatively low, patients requiring massive transfusions have a high mortality. One study at a level 1 trauma center found that only 1.7% of all their patients underwent a massive transfusion, defined as 10 units within 24 hours. An estimated 3% to 5% of civilian trauma patients and 10% of military trauma patients will receive a massive transfusion. While surgery is the most common use of major transfusion protocols (MTPs), trauma remains the best-studied category in massive transfusion. Patients potentially requiring massive transfusions can be seen across medicine from traumatic injuries, gastrointestinal bleeding, and obstetric catastrophes. This article reviews the current literature on massive transfusion protocols and explores the potential complications of this life-saving intervention. The goal of massive transfusion is to limit complications and to limit critical hypoperfusion while surgical hemostasis can be achieved. Massive transfusion is traditionally defined as transfusion of 10 units of packed red blood cells (PRBCs) within a 24 hour period.


 0 kommentar(er)
0 kommentar(er)
